https://www.pharmasources.com/news/73828.html
In-situ gel is a kind of liquid preparation, which will immediately transform to non-chemically crosslinked semisolid gel in the application site after administration. As a new formulation, in-situ gel has become one of the research hotspots in the field of preparation in recent years with huge potential in clinical application. Compared with common gel, in-situ gel has simple preparation technology. Before administration, in-situ gel is liquid preparation, which is convenient for administration with accurate dose. After administration, it will immediately transform to semisolid gel in vivo with long time residence in the application site and has good perform for sustained and controlled drug release. In addition, patients generally will not be uncomfortable after the use of this gel, representing its well acceptability and safety. In light of different formation mechanisms, in-situ gels can be divided into thermosensitive in situ gel, nanoemulsion in-situ gel and pH-sensitive in-situ gel.
Thermosensitive in situ gel: Thermosensitive in situ gel is made from thermosensitive polymers, which is in liquid below its lower critical solution temperature (LCST) and will be gelated when the ambient temperature reach or beyond LCST. It is the most widely studied and relatively mature sensitive gel at present. Matrix materials including poloxamer, chitosan, poly (N-isopropyl acrylamide) are common thermosensitive in situ gel.
Nanoemulsion in-situ gel: Nanoemulsion in-situ gel mainly uses the polymer solution formed by polysaccharide derivatives to undergo chemical reaction with a large number of cations such as K+, Na+, Ca2+ in the liquid environment in human body, and then undergo conformational change, thus forming gel at the application site. The commonly used materials are sodium alginate and deacetylated gellan gum.
PH-sensitive in-situ gel: PH-sensitive in-situ gel can receive or release protons from to the surrounding environment when the pH value of human body changes, which results in gelation reaction and slowly release drugs for a long time. The commonly used polymers include cellulose acetate phthalate, acrylic acid polymers (such as carbomer), chitosan and its derivatives.
Examples of in-situ gel preparations on the market
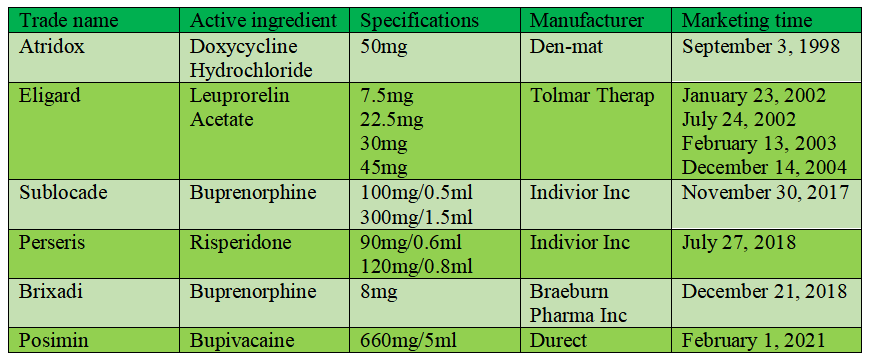
Atridox: Atridox is the first product approved for marketing with Atrigel technology. The product is light yellow or yellow viscous liquid. The preparation consists of 50mg doxycycline hydrochloride and 450mg polymer solution, which are packed in two syringes respectively. Before use, they are mixed evenly through an "axle tube", and then injected into the periodontal pocket of the patient with the needle, which can be continuously released in the periodontitis focus for one week. This product was listed abroad in 1998.
Eligard: Eligard (leuprorelin acetate) is a depot controlled release injection of luteinizing hormone releasing hormone (LHRH) agonist, which is used for palliative treatment of advanced prostate cancer. Eligard is a sterile suspension, which is prepared by Atrix Company's Atrigel drug sustained-release patented technology. It consists of two independent prefilled syringes, one of which is leuprorelin acetate powder, and the other is Atrigel system for reconstitution. After mixing and preparing the suspension within 30min before use, it was injected subcutaneously. After injection, NMP quickly diffuses into body fluid, and make the leuprorelin acetate loaded by PLGA become gel. Compared with the microsphere preparation of leuprorelin acetate, Eligard has a longer therapeutic effect with smaller dose. In December 2004, FDA approved the marketing of Eligard (45mg) of QLT Inc. of Canada, which was the first LHRH agonist approved by FDA to be used once every 6 months for the treatment of prostate cancer. Previously, several Eligard injections (with various specifications including 7.5mg, 22.5mg and 30mg) were approved for marketing.
 Back to List
Back to List